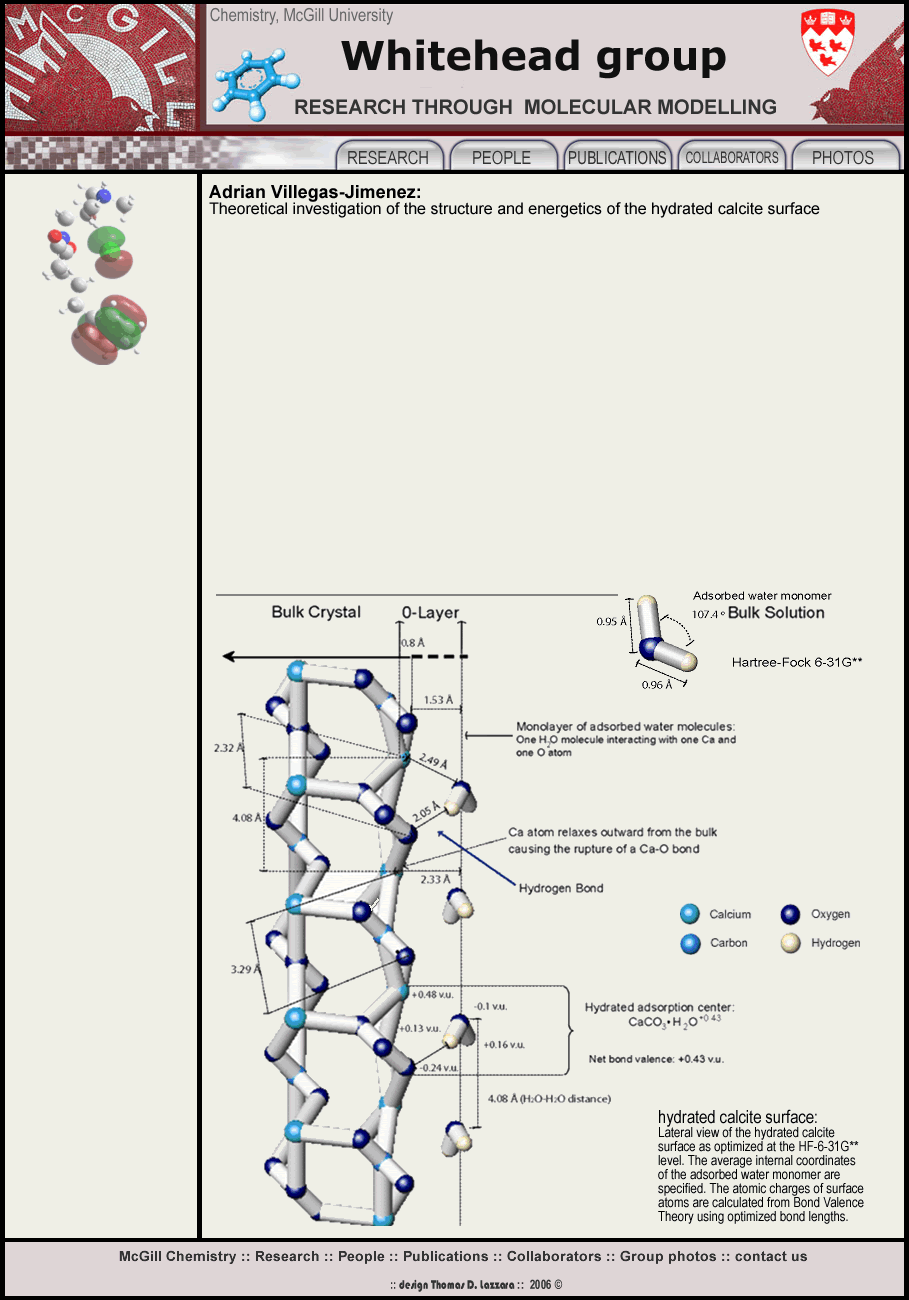
Earlier investigations have shown that the macroscopic reactivity of carbonate minerals in aqueous solutions is largely governed by reactions occurring at the mineral-water interface: water adsorption (hydration) is by far, the most fundamental reaction.
In aqueous solution, the cleavage plane is the most stable calcite surface, see the figure. This surface is an appropriate crystal face for investigating surface adsorption reactions.
We used Hartree-Fock ab initio methods to obtain insight into the three dimensional conformation and the energetics of the 1st and 2nd surface hydration layers. Using the cluster approach, we performed geometric optimizations on several charge-neutral CaCO3 clusters under dry and wet conditions.
Water adsorbs associatively on the CaCO3 surface and interacts mainly with Ca atoms, which relax outward from the surface causing the rupture of one of the bonds between a surface Ca and a sub-surface oxygen atom in the crystal lattice. A Ca-O bond is also broken at dry CaCO3 surfaces, but the degree of linear displacement of the surface Ca atoms is smaller than for the wet surface. This observation has never been reported before and might have important implications in the elucidation of a molecular theory of dissolution of CaCO3 in aqueous solutions.
Hydrogen bonding is established between one hydrogen of the water in the
first hydration layer and one surface oxygen atom, but no evidence is found
of hydrogen bonding between neighbouring adsorbed H2O
molecules in the first hydration layer. Conversely, water molecules
in the first hydration layer are hydrogen bonded to at least one H2O
located in the second hydration layer.
DOWNLOAD TEXT FILE
Whitehead group
Contacts
address:
Prof. M.A. Whitehead
Department of Chemistry
McGill University
Otto Maass Chemistry Building,
801 Sherbrooke St. West,
Room 352,
Montreal, Quebec,
H3A 2K6, Canada.
Phone:
office: (514) 398-6239
lab: (514) 398-6905
fax: (514) 398-3797
email:
tony.whitehead@mcgill.ca