Whitehead group
Contacts
address:
Prof. M.A. Whitehead
Department of Chemistry
McGill University
Otto Maass Chemistry Building,
801 Sherbrooke St. West,
Room 352,
Montreal, Quebec,
H3A 2K6, Canada.
Phone:
office: (514) 398-6239
lab: (514) 398-6905
fax: (514) 398-3797
email:
tony.whitehead@mcgill.ca
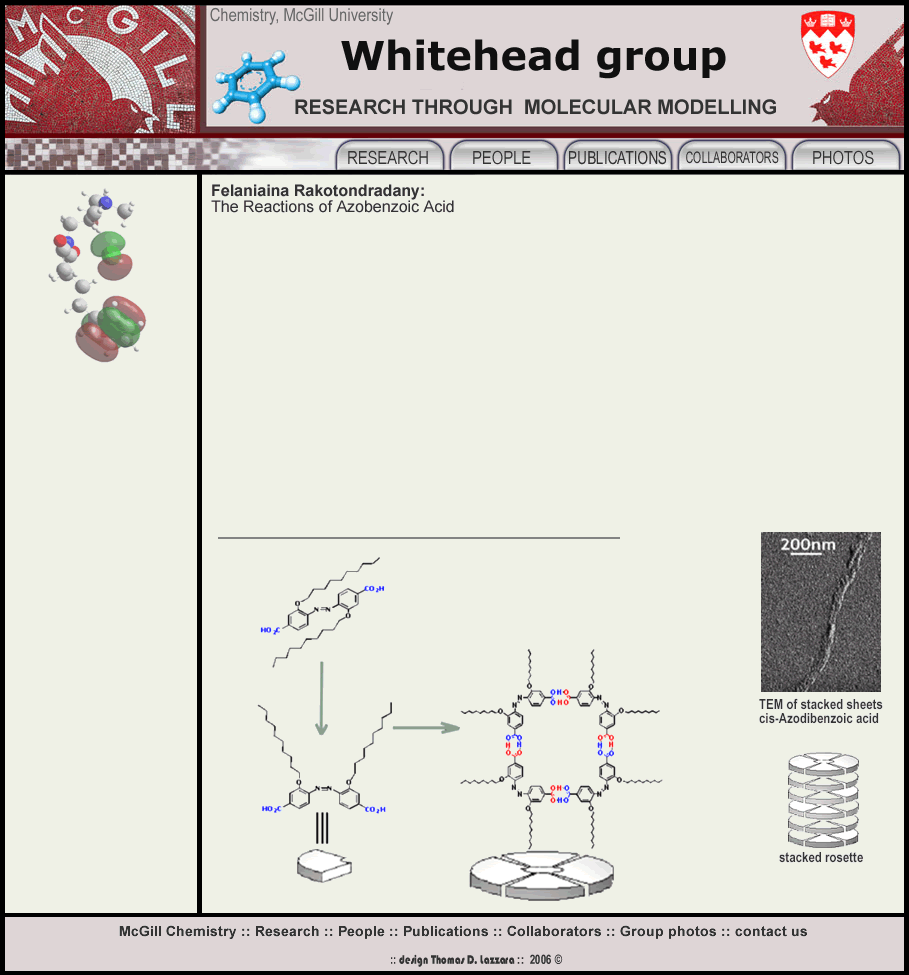
Novel discrete H-bonded aggregates are formed from the azodibenzoic acid building blocks, which are attractive for their ability to undergo a reversible cis-trans photoisomerization. Trans-azobenzene isomerizes upon irradiation with UV light. The resulting cis-azobenzene reverts to the trans isomer thermally or by irradiation with visible light. In the trans isomer, the two carboxylic groups para to the N=N linkage are aligned with respect to each other while, in the cis isomer, they are almost perpendicular to each other.
The cyclic tetramers self-organize into long, unidimensional rod-like sructures through stacking interactions. We have used a number of techniques, X-ray crystallography, X-ray powder diffraction, TEM, DLS to characterize these higher order structures. Computational studies were also used to better characterize the thermodynamics and predict the packing of these supramoleculecular structures.
This strcutural difference between the two isomers leads to different geometries by H-bonding: trans azodibenzoic acid aggregates linearly whereas its cis-counterpart in a cyclic fashion.We have demonstratd two different levels of organization occur. UV triggers the trans to cis isomerization, which changes the self-assembly of these molecules from linear tapes in the trans form to cyclic tetramers in the cis form.