Whitehead group
Contacts
address:
Prof. M.A. Whitehead
Department of Chemistry
McGill University
Otto Maass Chemistry Building,
801 Sherbrooke St. West,
Room 352,
Montreal, Quebec,
H3A 2K6, Canada.
Phone:
office: (514) 398-6239
lab: (514) 398-6905
fax: (514) 398-3797
email:
tony.whitehead@mcgill.ca
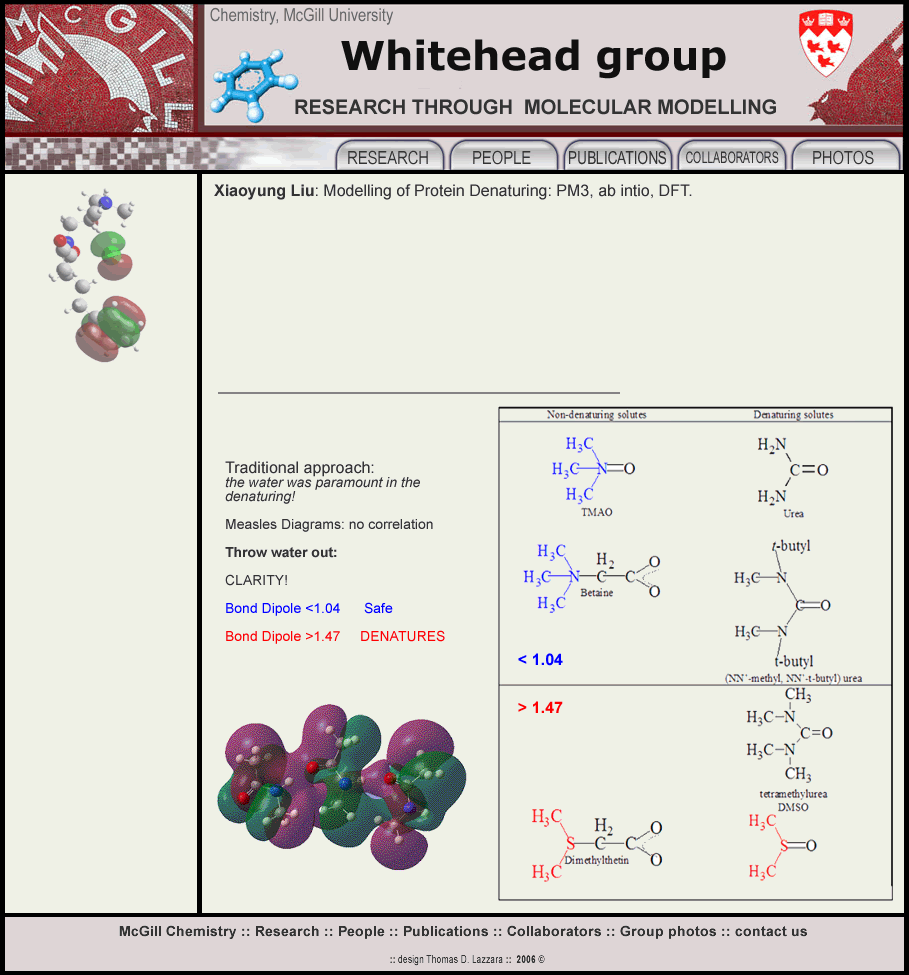
Attempts by Cecile Malardier to describe protein denaturing in terms of the disturbance in the three and more water shells surrounding the molecules failed, as well as relating the behaviour to the Molecular Dipole moment failed. A new approach; threw out the water and the Molecular Dipole Moment and used the gas phase molecular structure of the molecules and their Bond
Dipole Moments. A complete correlation of the Denaturing Molecules
having Bond Dipole Moments >1.47 and the non-denaturing Molecules <1.04. A sheet of amide molecules was minimized and then various molecules
inserted between the hydrogen bonded sheets: non-denaturing molecules
caused a distortion of the sheets forming weaker hydrogen bonds which
still held the sheets together: denaturing molecules caused the sheets
to re-arrange breaking the hydrogen bonds and disrupting the sheet. They unzipped the protein.
Xiaoyung threw out
the water, confirmed Cecile's values for the bond dipole moments of the molecules thought to denature or not denature. Modelling a protein by a tri-imide unit at the RF-STO-3G** level of theory, she showed that the hydrogen bonds which hold the protein rows and layers together were broken by the denaturing molecules, but only weakly disturbed by the non-denaturing molecules.